Arq. Bras. Cardiol. 2024; 121(6): e20230848
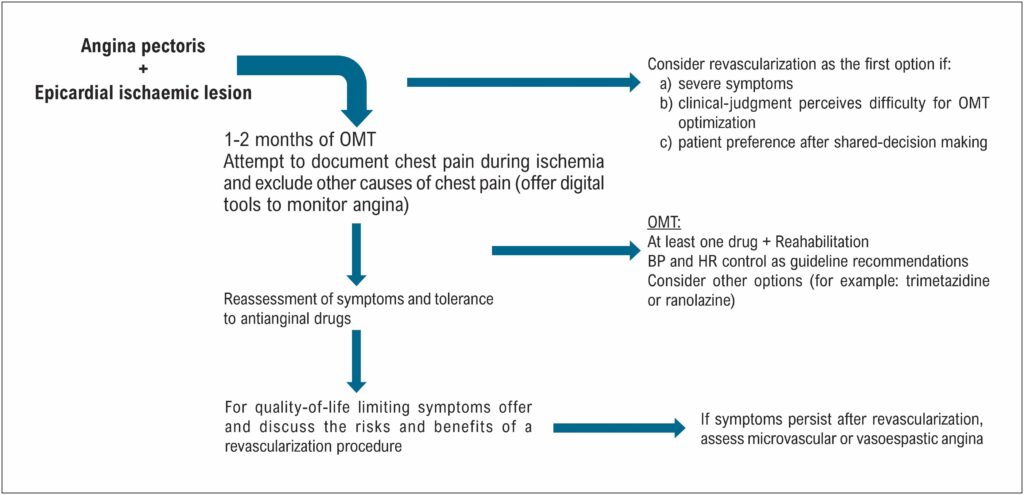
Angina Pectoris and Orbita 2 Trial: Reflections on the Future of Angina Treatment
The ORBITA trial
Although the above-mentioned studies have demonstrated some improvement in angina after myocardial revascularization, one of their major limitations is the open nature and a possible placebo effect. In this regard, the ORBITA study showed no benefit of PCI versus a placebo procedure on the primary end-point of an increase in exercise time. ORBITA was a well conducted study that demonstrated the possibility of a placebo component and lack of symptom improvement by PCI. However, several limitations could have influenced these results and have been discussed in the literature, such as the fact that it included patients with non-limiting symptoms, no ischemia in almost a quarter of the participants and short follow-up.
In 2023, the ORBITA 2 trial was finally published in NEJM and addressed for the first time the PCI as a monotherapy for angina relief at a placebo-controlled trial. It provides another piece of the puzzle of angina control and overcomes some of the first ORBITA limitations. ORBITA 2 was an investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial that was conducted at 14 sites in the United Kingdom and included 301 participants. Patients enrolled had angina or equivalent symptoms, anatomic evidence of severe coronary stenosis and objective evidence of ischemia based on noninvasive imaging or invasive coronary physiology testing. First, antianginal medications were discontinued (median number of antianginal drugs was only 1) and the participants were instructed to use a dedicated smartphone application to report the presence or absence of angina and the number of angina episodes daily. Patients also completed validated questionnaires on symptoms and quality of life. The Canadian Cardiovascular Society (CCS) functional classification was assessed, treadmill exercise test and dobutamine stress echocardiography were also performed. The patients then entered a two-week pre-randomization period during which they reported the number of episodes of angina daily, through the smartphone application. Patients were eligible to proceed to randomization if they reported at least one episode of angina during the symptom assessment phase.
[…]
740