Arq. Bras. Cardiol. 2025; 122(4): e20240589
Effectiveness and Safety of Edoxaban in the Routine Clinical Care of Atrial Fibrillation Patients in Brazil: Prospective 1-Year Follow-Up Study – EdoBRA
This Original Article is referred by the Short Editorial "Challenges in Performing Real-World Evidence Studies in Low-Middle Income Countries: the EdoBRA Study".
Abstract
Background
Edoxaban, an orally administered anticoagulant, has been shown to be safe and effective in preventing stroke in atrial fibrillation (AF) patients. Given its widespread use since approval, evaluating edoxaban’s real-world performance in the Brazilian clinical context is crucial.
Objective
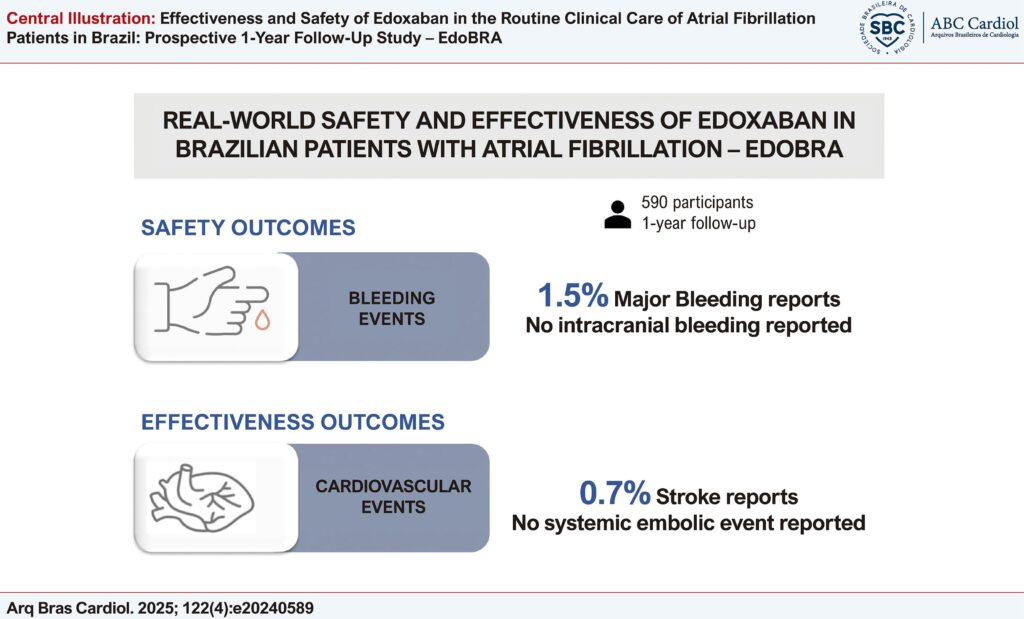
The study aimed to report the one-year safety and effectiveness of edoxaban in AF patients in Brazil.
Methods
EdoBRA is a multi-center, prospective, observational investigation conducted across 30 Brazilian research sites. Bleeding events were considered as safety measures and cardiovascular events were considered for effectiveness measures. Descriptive analyses were performed. Kaplan-Meier curves were generated for time-to-event analysis and a 95% confidence interval was used as appropriate.
Results
Among the 705 enrolled participants, 590 were included in the analysis for having at least one follow-up or one reported event. Mean (±SD) CHA2DS2-VASc risk score was 3 (3.3 ± 1.6) and the mean HAS-BLED risk score was 2 (1.8 ± 1.2). During the one-year follow-up period, nine major bleedings events were reported, including five cases of gastrointestinal bleeding (IP 0.85 [95% CI =0.82; 0.88]). Among the cardiovascular events recorded (N = 68), there were four stroke events (IP 0.68 [CI 95% 0.65;0.71]), one transient ischemic attack (IP 0.17 [CI 95% (0.16;0.18]) and 1 event was Venous Thromboembolic Events (IP 0.17 [CI 95% (0.16;0.18]). No systemic embolic event was exhibited by any patient.
Conclusion
In an elderly population with several comorbidities routinely treated with edoxaban for AF, the rates of cardiovascular event and major bleeding were low.
Keywords: Anticoagulants; Atrial Fibrillation; Hemorrhage; Stroke
776