Arq. Bras. Cardiol. 2025; 122(4): e20240568
Patisiran Treatment in the Brazilian Subpopulation of the Phase 3 APOLLO-B Study in Transthyretin Amyloidosis with Cardiomyopathy: Post Hoc Analysis
This Original Article is referred by the Short Editorial "Patisiran in the Treatment of Cardiac Amyloidosis: Good for the World and Good for Brazil".
Abstract
Background
Patisiran rapidly knocked down transthyretin and preserved functional capacity in patients with transthyretin amyloidosis with cardiomyopathy (ATTR-CM) in the global Phase 3 APOLLO-B study (NCT03997383).
Objectives
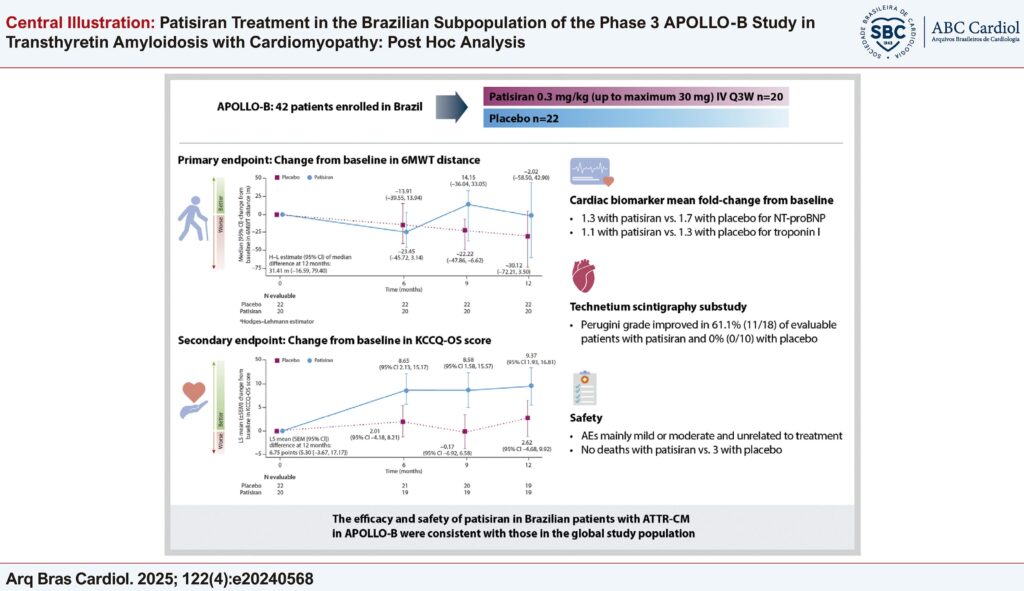
To evaluate patisiran efficacy and safety in post hoc analysis of the Brazilian subpopulation of APOLLO-B.
Methods
Patients were randomized 1:1 to patisiran 0.3 mg/kg or placebo every 3 weeks for 12 months. The primary endpoint was the change from baseline (CFB) in functional capacity (6-minute walk test [6MWT]) at Month 12. Secondary endpoints included CFB to Month 12 in the Kansas City Cardiomyopathy Questionnaire-Overall Summary (KCCQ-OS) score. Exploratory endpoints included CFB in cardiac biomarkers and Perugini grade of cardiac uptake during technetium-99m scintigraphy.
Results
Forty-two patients enrolled in Brazil (patisiran, n=20; placebo, n=22). Patisiran showed benefit in 6MWT and KCCQ-OS scores vs. placebo; CFB (95% confidence interval [CI]) in 6MWT (median) and KCCQ-OS scores (least squares mean) was –2.0 m (–58.5, 42.9) and 9.37 (1.93, 16.81) points with patisiran vs. –30.1 m (–72.2, 3.5) and 2.62 (–4.68, 9.92) points for placebo. For cardiac biomarkers, the mean fold-change from baseline (95% CI) for N-terminal prohormone B-type natriuretic peptide and troponin I was 1.31 (1.06, 1.61) and 1.12 (0.94, 1.34) for patisiran, and 1.71 (1.39, 2.10) and 1.28 (1.08, 1.53) for placebo, respectively. Perugini grade improved in 11/18 (61.1%) and 0/10 evaluable patients with patisiran and placebo, respectively. There were no deaths in the patisiran group vs. 3 in the placebo group.
Conclusion
The efficacy and safety of patisiran in Brazilian patients with ATTR-CM in APOLLO-B were consistent with those in the global study population. Findings are descriptive due to the small number of patients.
521