Arq. Bras. Cardiol. 2020; 115(3): 515-524
Percutaneous Transseptal Bioprosthetic Implantation in Failed Prosthetic Surgical Mitral Valve – Brazilian Multicenter Experience
This Original Article is referred by the Short Editorial "Transseptal, Transcatheter Mitral Valve-In-Valve Replacement: Ready for Prime Time Treatment of Bioprosthetic Valve Failure in Brazil?".
Abstract
Background
Percutaneous intervention in patients with bioprosthetic mitral valve dysfunction is an alternative to conventional surgical treatment.
Objectives
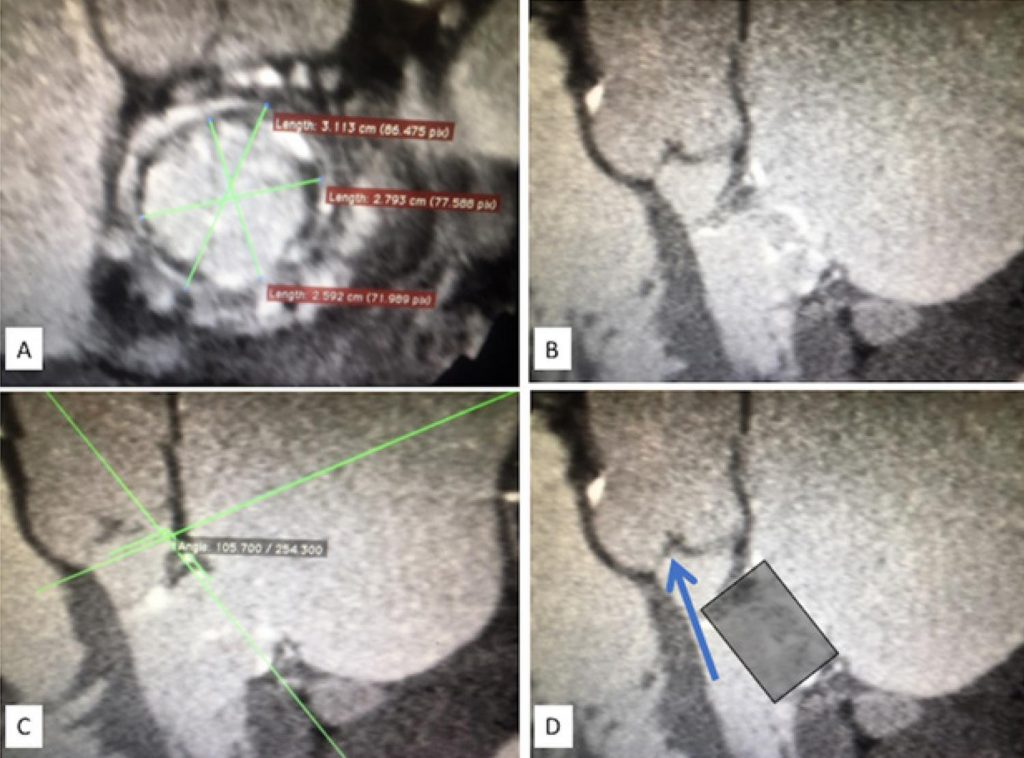
To report the first Brazilian experience with transseptal transcatheter bioprosthetic mitral valve-in-valve implantation (transseptal-TMVIV).
Methods
Patients with surgical bioprosthetic dysfunction submitted to transseptal-TMVIV in 12 Brazilian hospitals were included. The significance level adopted was p<0.05.
Results
From June/2016 to February/2019, 17 patients underwent transseptal-TMVIV. Their median age was 77 years (IQR,70-82) and median Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score was 8.7% (IQR,7.2-17.8). All patients had limiting symptoms of heart failure (FC≥III) and 5 (29.4%) had undergone more than one previous thoracotomy. Transseptal-TMVIV was successful in all patients. Echocardiographic assessment showed a significant reduction in mean mitral valve gradient (pre-intervention, 12±3.8 mmHg; post-intervention, 5.3±2.6 mmHg; p<0.001), in addition to an increase in mitral valve area (pre-intervention, 1.06±0.59 cm2; post-intervention, 2.18±0.36 cm2; p<0.001) sustained for 30 days. There was a significant and immediate reduction in the pulmonary artery systolic pressure, with an additional reduction in 30 days (pre-intervention, 68.9±16.4 mmHg; post-intervention, 57.7±16.5 mmHg; 30 days, 50.9±18.7 mmHg; p<0.001). During follow-up (median, 162 days; IQR, 102-411), significant clinical improvement (FC≤II) was observed in 87.5% of the patients. One patient (5.9%) had left ventricular outflow tract (LVOT) obstruction and died right after the procedure, and another died at 161 days of follow-up.
Conclusion
The first Brazilian experience with transseptal-TMVIV shows the safety and effectivity of the new technique. The LVOT obstruction is a potentially fatal complication, reinforcing the importance of patients’ selection and of procedural planning. (Arq Bras Cardiol. 2020; 115(3):515-524)
1,316