Arq. Bras. Cardiol. 2023; 120(3): e20220431
Rivaroxaban in Outpatients with Mild or Moderate COVID-19: Rationale and Design of the Study CARE (CARE – Coalition COVID-19 Brazil VIII)
This Original Article is referred by the Short Editorial "Brazilian Initiatives Head Strong Scientific Cooperation to Address COVID-19 Issues: The Case of Coalition COVID-19 Brazil".
Abstract
Background
Previous studies have demonstrated a high risk of arterial and venous thromboembolic events as a consequence of direct viral damage to endothelial cells by SARS-CoV-2 and a procoagulant milieu due to increased biomarkers, such as D-dimer, fibrinogen, and factor VIII. Although randomized controlled trials of antithrombotic therapies have been conducted in hospitalized patients, few have evaluated the role of thromboprophylaxis in an outpatient setting.
Objective
To assess whether antithrombotic prophylaxis with rivaroxaban reduces the risk of venous or arterial thrombotic events, invasive ventilatory support, and death in COVID-19 outpatients.
Methods
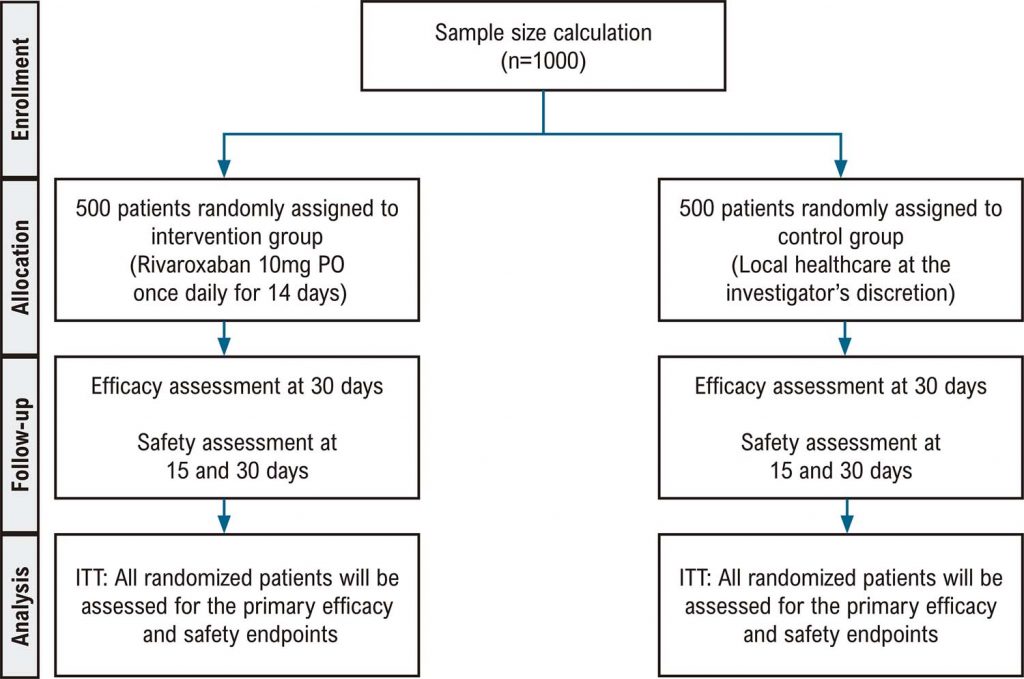
The COVID Antithrombotic Rivaroxaban Evaluation (CARE) study, a multicenter, randomized, open-label, controlled trial of rivaroxaban 10 mg once daily for 14 days or local standard treatment alone to prevent adverse outcomes, is registered in clinicaltrials.gov (NCT04757857). The inclusion criteria are adults with confirmed or suspected SARS-CoV-2 infection and mild or moderate symptoms without indication for hospitalization, within 7 days of symptom onset, and 1 risk factor for COVID-19 complication (> 65 years, hypertension, diabetes mellitus, asthma, chronic obstructive pulmonary disease or other chronic lung diseases, smoking, immunosuppression, or obesity). The primary composite endpoint, which includes venous thromboembolism, invasive mechanical ventilation, major acute cardiovascular events, and mortality within 30 days of randomization, will be assessed according to the intention-to-treat principle. All patients will provide informed consent. A significance level of 5% will be used for all statistical tests.
Results
Major thrombotic and bleeding outcomes, hospitalizations, and deaths will be centrally adjudicated by an independent clinical events committee blinded to the assigned treatment groups.
Conclusion
The CARE study will provide relevant and contemporary information about the potential role of thromboprophylaxis in outpatients with COVID-19.
2,060