Arq. Bras. Cardiol. 2025; 122(4): e20250335
Patisiran in the Treatment of Cardiac Amyloidosis: Good for the World and Good for Brazil
This Short Editorial is referred by the Research article "Patisiran Treatment in the Brazilian Subpopulation of the Phase 3 APOLLO-B Study in Transthyretin Amyloidosis with Cardiomyopathy: Post Hoc Analysis".
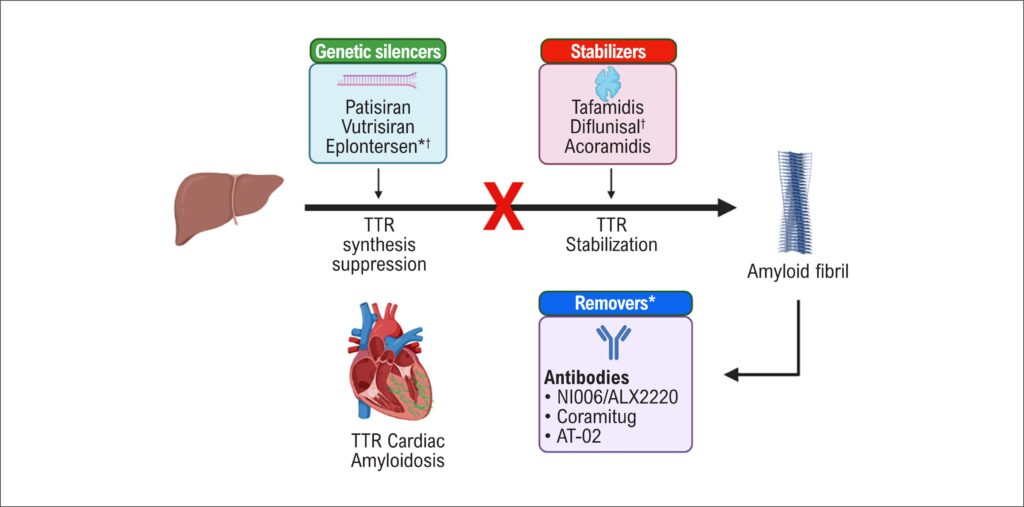
Patisiran is an RNA interference therapeutic agent used in the treatment of transthyretin-mediated amyloidosis (ATTR amyloidosis), a condition characterized by the accumulation of misfolded transthyretin (TTR) protein amyloid fibrils in tissues.– As shown in , the mechanism of action differs from previous drugs such as tafamidis, which promotes the stabilization of the TTR protein, rather than the synthesis inhibition., Patisiran uses a mechanism involving small interfering RNA to specifically target the messenger RNA (mRNA) responsible for producing the TTR protein in the liver. It activates the RNA-induced silencing complex, which promotes the degradation of the TTR mRNA. By degrading TTR mRNA, patisiran effectively reduces the synthesis of TTR protein within the liver. This leads to decreased levels of both normal and mutant forms of TTR.–
The effects of patisiran in cardiac amyloidosis were assessed in the APOLLO B study, which was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial involving 360 patients diagnosed with hereditary or wild-type ATTR amyloidosis with cardiomyopathy, aimed at assessing treatment effects on cardiac function and quality of life. The primary endpoint was a change from baseline in the functional capacity as assessed by the 6-minute walk test (6MWT) at 12 months. The walk distance in the 6MWT decreased over time in both groups, but it was attenuated in the patisiran group (mean change −8.15 vs −21.35 m, in the patisiran and placebo group, respectively). Quality of life as assessed by the Kansas City Cardiomyopathy Questionnaire-Overall Summary (KCCQ-OS) improved in the patisiran group and declined in the placebo group. In a secondary endpoint analysis, no difference was observed in a combined endpoint of death from any cause, cardiovascular events, or change in the 6MWT. An explanatory analysis found that patisiran attenuated the increase in the cardiac biomarkers NT-proBNP and troponin I.
[…]
Keywords: Cardiac Amyloidosis; Genetic Silencers; Transthyretin; Treatment
141